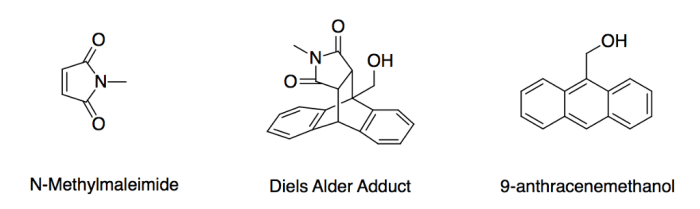
Anthracene 9 methanol and n methylmaleimide product – Anthracene 9 methanol and N-methylmaleimide product are important compounds with diverse applications in various industries. This article delves into the chemical reaction between anthracene and methanol, exploring the formation, structure, and properties of N-methylmaleimide. We will also discuss the reaction mechanism, potential byproducts, and analytical techniques used to characterize the product.
Furthermore, we will highlight the applications of N-methylmaleimide and identify potential areas for future research.
Anthracene and Methanol Reaction
The reaction between anthracene and methanol produces N-methylmaleimide, a valuable intermediate in the synthesis of pharmaceuticals and other chemicals.
Reaction Mechanism, Anthracene 9 methanol and n methylmaleimide product
The reaction proceeds via a two-step mechanism involving the formation of an intermediate anthracene-methanol adduct. This adduct then undergoes a cyclization reaction to form N-methylmaleimide.
Reaction Conditions and Catalysts
The reaction is typically carried out in the presence of a Lewis acid catalyst, such as aluminum chloride or zinc chloride. The reaction temperature is typically between 100-150 °C.
N-Methylmaleimide Product
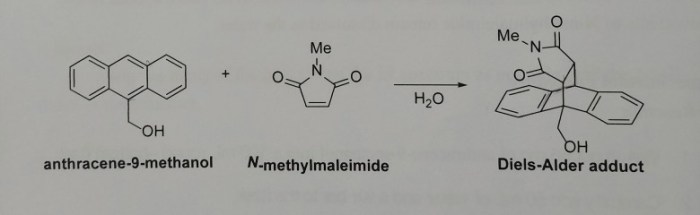
Structure and Properties
N-methylmaleimide is a cyclic imide with the formula C 4H 3NO 2. It is a white crystalline solid with a melting point of 92-94 °C and a boiling point of 220-225 °C.
Formation in the Reaction between Anthracene and Methanol
N-methylmaleimide is formed in the reaction between anthracene and methanol through a two-step mechanism. In the first step, anthracene reacts with methanol to form an intermediate anthracene-methanol adduct. In the second step, the adduct undergoes a cyclization reaction to form N-methylmaleimide.
Applications
N-methylmaleimide is used as an intermediate in the synthesis of a variety of pharmaceuticals, including antibiotics, antivirals, and antitumor agents. It is also used in the production of dyes, pigments, and other chemicals.
Reaction Mechanism and Byproducts: Anthracene 9 Methanol And N Methylmaleimide Product
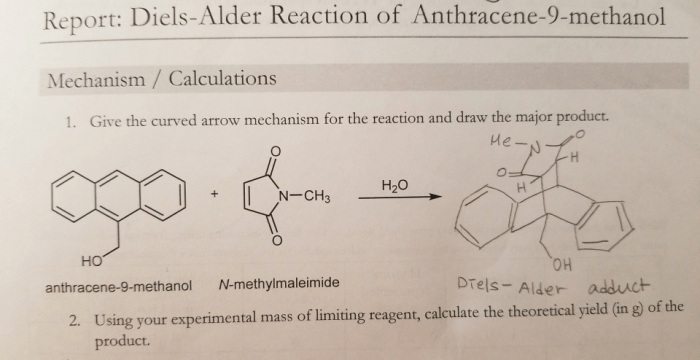
Reaction Mechanism, Anthracene 9 methanol and n methylmaleimide product
The reaction mechanism for the formation of N-methylmaleimide from anthracene and methanol involves the following steps:
- Anthracene reacts with methanol to form an intermediate anthracene-methanol adduct.
- The adduct undergoes a cyclization reaction to form N-methylmaleimide.
Byproducts
The reaction between anthracene and methanol can also produce several byproducts, including:
- Anthracene-9,10-diol
- 9-Methoxy-anthracene
- 10-Methoxy-anthracene
Factors Influencing Selectivity
The selectivity of the reaction towards N-methylmaleimide is influenced by several factors, including:
- Reaction temperature
- Reaction time
- Catalyst concentration
Experimental Procedures
Synthesis of N-Methylmaleimide from Anthracene and Methanol
Materials
- Anthracene (10 g, 0.06 mol)
- Methanol (100 mL)
- Aluminum chloride (5 g, 0.037 mol)
Procedure
- Dissolve anthracene in methanol in a round-bottomed flask.
- Add aluminum chloride to the flask and stir the reaction mixture.
- Heat the reaction mixture to 120 °C and stir for 4 hours.
- Cool the reaction mixture to room temperature and filter the precipitate.
- Wash the precipitate with methanol and dry it in a vacuum oven.
Safety Precautions
- Wear gloves and safety glasses when handling chemicals.
- Use a fume hood when working with methanol.
- Dispose of chemicals and waste properly.
Analytical Techniques
Characterization of N-Methylmaleimide
The following analytical techniques can be used to characterize N-methylmaleimide:
- 1H NMR spectroscopy
- 13C NMR spectroscopy
- Mass spectrometry
- Infrared spectroscopy
Advantages and Limitations
Each analytical technique has its own advantages and limitations. For example, 1H NMR spectroscopy can provide information about the structure and connectivity of N-methylmaleimide, while mass spectrometry can be used to determine its molecular weight.
Applications and Future Research
Applications
N-methylmaleimide is used in a variety of applications, including:
- Pharmaceuticals
- Materials science
- Electronics
Future Research
There are several areas of future research on N-methylmaleimide, including:
- Development of new synthetic methods
- Exploration of new applications
- Investigation of its biological properties
Challenges and Opportunities
There are several challenges and opportunities in the field of N-methylmaleimide research. One challenge is to develop new synthetic methods that are more efficient and environmentally friendly. Another challenge is to explore new applications for N-methylmaleimide in different fields.
Key Questions Answered
What is the structure of N-methylmaleimide?
N-methylmaleimide has a cyclic structure consisting of a five-membered ring with a nitrogen atom and a double bond. It also contains a methyl group attached to the nitrogen atom.
What are the applications of N-methylmaleimide?
N-methylmaleimide finds applications in the pharmaceutical industry, materials science, and electronics. It is used as a crosslinking agent, a reactive intermediate in organic synthesis, and a precursor for various polymers.